- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Grams per litre
Overview
The concentration of a solution (formed from the addition of a solute to a solvent) is given by:

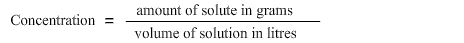
Units are then grams/litre (g/L).
It may also be helpful to think of it as the number of grams of substance needed for each litre of solution.
Example
Find the concentration in g/L when 21g of Sodium Chloride NaCl is dissolved in 350mL of water.
The video below shows the systematic approach to solving the above problem.
![]() This video contains sound.
This video contains sound.
The process of solving problems like these can be simplified by following a series of steps.
The first step is to extract from the problem statement the information that has been provided and the information being asked for.
The information we are provided with is:
We have 21 grams of NaCl and 350 mL of water.
The information we are asked for is:
Concentration in grams/litre of the solution resulting from dissolving Sodium Chloride in water.
The second step is to identify the formula to find out the information being asked for.
In this problem we are being asked to find concentration in g/L. Hence the formula to calculate concentration is:
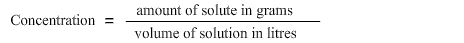
The third step is to match the information provided in the problem statement with the formula. As NaCl is dissolved in water, therefore NaCl is the solute i.e. 21 grams and water is the solution i.e. 350 millilitres. Hence, we have both pieces of information required to calculate concentration.
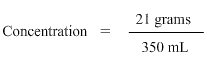
The fourth step is to ensure that the information being used in the formula is in the correct units. In this problem we have been asked to find the concentration in grams/litre so the solute should always be in grams and the solution should always be in litres. Sodium Chloride is in grams however water is in millilitres and not litres. Hence we need to convert water from millilitres to litres.
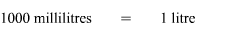
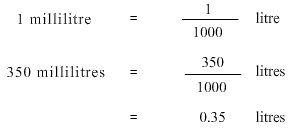
We now have both the values in the correct units, so we go ahead with the calculations. We get,
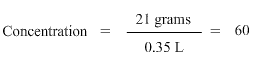
Do not forget to add unit to the answer. The unit of concentration is g/L, hence the answer to the exercise is 60 g/L.