- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Moles per litre
Overview
The concentration of a solution (formed from the addition of a solute to a solvent) is given by:

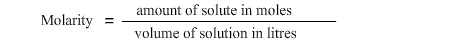
The above formula is sometimes written as:


where C is Concentration, n is the amount of solute in moles and V is the volume of solution in litres. This formula can be rearranged to give any one of C, n, V once the other two quantities are known i.e.

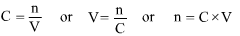
Units of concentration are moles per litre (mol L-1 or mol/L). With these units, concentration is often called Molarity, written M i.e. 0.1M = 0.1mol/L etc.
Note that if the amount of solute is given in grams, you need to convert it into moles. Figure 1 shows the relationship between concentration and the amount of solute in grams.
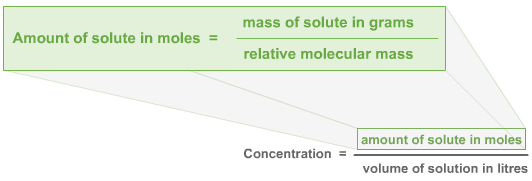
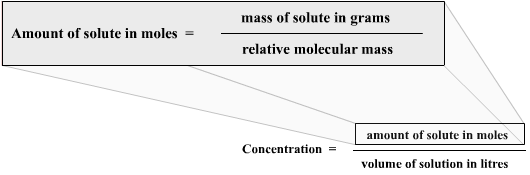
Figure 1: Relationship between concentration and amount of solute in grams
It may also be helpful to think of molarity as the number of moles of substance needed for each litre of solution.
For low concentrations, it is often more convenient to express concentration in mM (10-3M) and μM (10-6M).
Molarity is the most widely used unit for concentrations of solutions in biology and chemistry.