- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Percent solutions
Overview
There are two types of percent concentration. These are % concentration (w/v) and % concentration (v/v).
% concentration (w/v), where w/v means weight of substance per volume of solvent and % means parts per hundred, is used with a dry reagent.

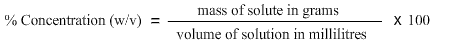
It may also be helpful to think of it as the number of grams of substance needed for each 100mL of solution.
For water based solutions remember 1 mL of water has a mass of 1 g.
% concentration (v/v), where v/v means volume of solute per volume of solution, is used with liquid reagents.
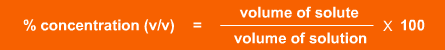
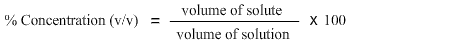
(Note it is important to use the same units of volume for each substance).
It may also be helpful to think of it as the number of mL of the solute for each 100 mL of final solution.