- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Serial dilution
Overview
A serial dilution is simply a series of simple dilutions, which has the effect of amplifying the dilution factor quickly.
The video below explains the concept of serial dilution.
![]() This video contains sound.
This video contains sound.
Let's suppose that we have a Copper Sulphate solution with a concentration of 0.5 Molar. We take 10 mL of this solution and add it to 90 mL of distilled water, resulting in a total volume of 100 mL. The concentration of the resulting solution will be reduced by 10 times the original concentration i.e. 0.5 divided by 10 which is equal to 0.05. So the resulting solution has a concentration of 0.05 Molar. Now if we take 10 mL of this 0.05 Molar solution and add it to another 90 mL of water, resulting again in a total volume of 100 mL, then the concentration of this solution will be reduced by a factor of 10 as well. This will be 0.05 divided by 10 i.e. 0.005 Molar. This process is called serial dilution.
The concentration of initial solution is 0.5 Molar. The concentration of solution after 2 dilutions is 0.005 Molar. Thus the combined effect of the two dilutions is to achieve a dilution factor of 100.
In a serial dilution, the total dilution factor at any point is the product of the individual dilution factors at each step i.e.
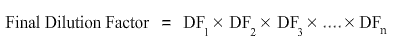
where n is the number of serial dilutions.
As dilution factor in each of the two dilution steps was 10, therefore the final dilution factor is going to be 10 times 10 which is equal to 100.
Serial dilutions are commonly performed with individual dilution factors of 10 and 100.