- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Moles per litre
Activity 2
Find the molarity of a solution with a volume of 200mL containing 12g of Hydrogen Fluoride (HF).
Try to attempt this problem yourself. You may wish to follow the steps identified in the example provided in the previous section to solve problems like these. Type-in your answer (digits only) in the text box given below and then check your answer by clicking the 'Check Answer' button.
M r for Hydrogen Fluoride is 20 and remembering the formula:
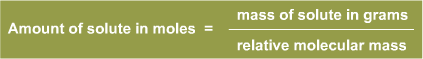
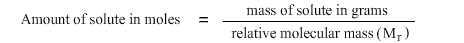
the amount of Hydrogen Fluoride in moles is 12 ÷ 20 = 0.6 moles.
Also, the solution should be in litres. Using the conversion:

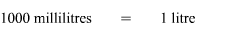
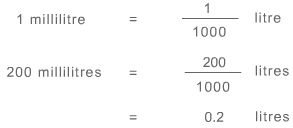
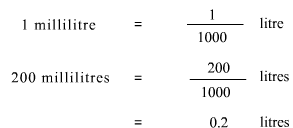
Amount of solute is 0.6 moles and volume of solution is 0.2 litres. As the formula for determining molarity or concentration of a solution is:

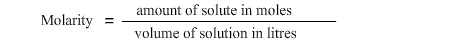
therefore by placing the values in the formula we get molraity equal to 0.6 moles ÷ 0.2 litres = 3M.
You can go to another section of this resource if you have got the answer right, or else, the video below shows a detailed step by step approach to solve the above problem.
![]() This video contains sound.
This video contains sound.