- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Dilution
Activity 2
You have a small amount of an expensive stock solution with concentration 80 mg/mL and need to make 240 µL of solution having 20 mg/mL concentration. What volume of stock solution is required?
Try to attempt this problem yourself. You may wish to follow the steps identified in the example provided in the previous section to solve problems like these. Type-in your answer (digits only) in the text box given below and then check your answer by clicking the 'Check Answer' button.
Use C1V1 = C2V2 with subscript 1 for stock solution, 2 for new solution.
We have C1 = 80 mg/mL, C2 = 20 mg/mL, V2 = 240 µL and need V1
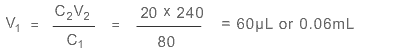
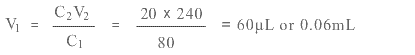
Hence you would use 60 µL of stock solution diluted with 180 µL of solvent. Remember to subtract the amount of stock solution from the required quantity to calculate the amount of solvent.
You can go to another section of this resource if you have got the answer right, or else, the video below shows a detailed step by step approach to solve the above problem.
![]() This video contains sound.
This video contains sound.