- Units & Notation
- Moles per litre
- Grams per litre
- Percent solutions
- Parts per million
- Practice problems
Percent solutions
Example 2
Find the percent concentration (v/v) when 15 mL of glycerol is added to a buffer to make a total volume of 200 mL.
The video below shows the systematic approach to solving the above problem.
![]() This video contains sound.
This video contains sound.
The process of solving problems like these can be simplified by following a series of steps.
The first step is to extract from the problem statement the information that has been provided and the information being asked for.
The information we are provided with is:
We have 15 mL of glycerol and 200 mL of solution resulting from dissolving glycerol in the buffer.
The information we are asked for is:
Percent concentration (v/v) resulting from dissolving glycerol in buffer.
The second step is to identify the formula to find out the information being asked for.
In this problem we are being asked to find percent concentration (v/v). Hence the formula to calculate percent concentration (v/v) is:
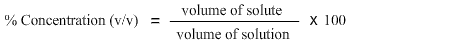
The third step is to match the information provided in the problem statement with the formula. As glycerol is dissolved in buffer, therefore glycerol is the solute i.e. 15 ml and the total volume of the resulting solution is 200 mL. Hence, we have both pieces of information required to calculate concentration.
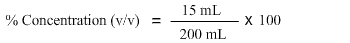
The fourth step is ensure that both the volume of solute and the solution are in the same units, whether these are millilitres, litres etc. In this case, both the solute and the solution have the same units, so we go ahead with the calculations.
15 mL divided by 200 mL gives 0.075.
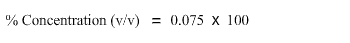
0.075 multiplied by 100 is 7.5. Hence the percent concentration (v/v) when 15mL of glycerol is added to a buffer to make a total volume of 200 mL is 7.5%.